The accumulation of greenhouse gases in the atmosphere is the main driver of global warming. The consequences of this include droughts, heavy rainfall, storms, and flooding. Melting ice masses also raise sea levels. But why exactly are greenhouse gases harmful? What types exist? And where do they originate? This article provides the answers.
What Do Greenhouses and Earth’s Climate Have in Common?
In a greenhouse, electromagnetic radiation from the sun passes through the transparent walls and roofs, mostly as visible light. When this radiation hits interior surfaces, they absorb it and heat up.
The heat becomes trapped, raising the temperature of the air and objects inside the greenhouse. Greenhouse gases function similarly. They allow shortwave solar radiation to pass through but partially absorb or reflect the longwave thermal radiation emitted by the Earth’s surface.
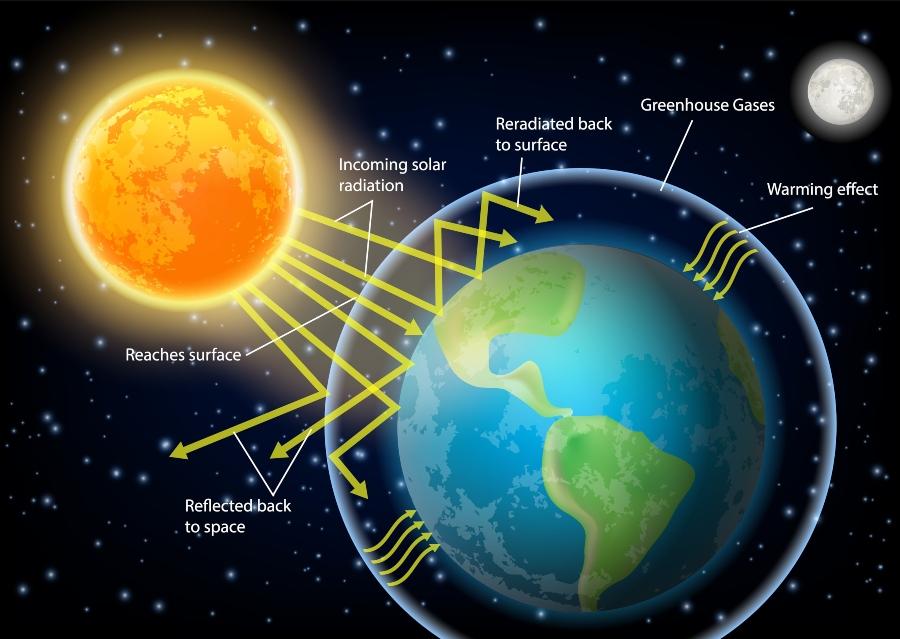
© Siberian Art – stock.adobe.comThis distinguishes them from the main components of air, namely nitrogen and oxygen, which let both short and long waves pass through almost without obstruction. The reason lies in molecular structure: Heat is the motion of atoms within a substance. Nitrogen and oxygen molecules consist of only two atoms and can only vibrate in limited ways. Greenhouse gases, by contrast, have three or more atoms and can vibrate in more complex patterns, which allows them to absorb or reflect a wider range of wavelengths and thus trap more heat.
Why Are Greenhouse Gases Harmful?
The most important greenhouse gases are water vapor and carbon dioxide. Both have existed in Earth’s atmosphere long before industrialization. Water vapor enters the atmosphere via evaporation and remains there until it condenses into clouds and returns to the ground as rain, snow, or hail.
Carbon dioxide is released through the respiration of living organisms. Plants absorb it and use it as a nutrient. Over millions of years, this exchange created a natural balance that maintained temperatures on our planet within a habitable range.
Industrialization, transportation, and intensive agriculture have disrupted this balance. Over the past 200 years, human activity has significantly increased carbon dioxide concentrations and introduced other greenhouse gases into the atmosphere. The result has been rising temperatures. The warmer air holds more moisture, and a thawing permafrost releases even more gases.
This creates a feedback loop that intensifies the greenhouse effect. The consequences include extreme weather events, melting ice masses, and infrastructure damage, which in turn impact supply chains for raw materials and energy. Rivers and lakes dry up during droughts, affecting industries such as chemicals and energy that depend on water for cooling. When water levels drop and temperatures rise, discharging wastewater into rivers becomes unsustainable. In some cases, discharge bans must be issued to protect ecosystems—bringing chemical production to a standstill. In short: Emitting greenhouse gases, in the chemical industry and beyond, is ultimately self-destructive.
What Greenhouse Gases Exist?
In addition to water vapor and carbon dioxide, other major contributors to the greenhouse effect include methane, nitrous oxide, hydrofluorocarbons (HFCs), perfluorocarbons (PFCs), sulfur hexafluoride (SF₆), and nitrogen trifluoride (NF₃). HFCs and PFCs belong to the group known as fluorinated gases (F-gases). In the United States, emissions of these substances from building operations, transportation, agriculture, and industry are measured and reported annually through the Environmental Protection Agency’s Greenhouse Gas Reporting Program. This monitoring ensures transparency and helps shape both domestic climate policy and international commitments. Other climate-relevant substances include ground-level ozone, nitrogen dioxide (NO₂), sulfur dioxide (SO₂), and ammonia (NH₃).

Thanks to progress in chemistry, emissions of many of these substances have steadily decreased across all sectors. However, their climate impact varies depending on how much thermal radiation they can absorb or reflect—and how long they remain in the atmosphere. This is measured the Global Warming Potential (GWP), which quantifies the warming effect of a substance over 100 years relative to carbon dioxide.
The GWP values (in CO₂ equivalents) are as follows:
- Carbon dioxide (CO₂): 1
- Methane (CH₄): 25
- Nitrous oxide (N₂O): 298
- Nitrogen trifluoride (NF₃): 17,200
- Sulfur hexafluoride (SF₆): 22,800
These high values illustrate that even trace amounts of some gases can significantly accelerate global warming.
Where Are Greenhouse Gases Generated?
Carbon Dioxide
CO₂ emissions make up the largest share of all greenhouse gases. They are primarily released during energy generation from fossil fuels and fuel combustion in vehicle engines. The chemical industry is particularly energy-intensive. A significant share of its greenhouse gas emissions results from electricity use and from generating steam and hot water for heating. Additionally, many chemical processes release CO₂—for example, the production of soda ash, ammonia, and cement. Other large industrial sources include steelmaking and building operations.

Methane
Methane forms when organic matter is broken down by microorganisms in the absence of oxygen. Major sources include agriculture, sewage treatment plants, and landfills.
Nitrous Oxide
Nitrous oxide, also known as dinitrogen monoxide (N₂O), is produced when nitrogen-based compounds break down in soil—especially synthetic fertilizers and manure. That makes agriculture, particularly factory farming, a key contributor. In the chemical industry, large quantities of N₂O are released during the production of caprolactam, adipic acid, and nitric acid. Caprolactam and adipic acid are used to make plastics; nitric acid is a basic chemical feedstock in many applications.
To reduce emissions, N₂O is often captured and thermally decomposed in an additional processing step.
Fluorinated Hydrocarbons (F-Gases)
F-gases are synthetic chemicals used primarily as propellants, refrigerants in air conditioning systems, and fire suppression agents. Their use is regulated by two specific F-gas regulations. To minimize emissions, systems are subject to regular leak inspections and must be properly decommissioned. Reclaiming refrigerants from scrapped equipment also helps reduce emissions.

Sulfur Hexafluoride (SF₆)
SF₆ is a very dense, inert gas used as an insulator in electrical systems. It is also used in soundproofing windows and in semiconductor manufacturing. Emissions can be minimized by using sealed, hermetic systems.
Nitrogen Trifluoride (NF₃)
NF₃ is widely used in semiconductor manufacturing. It serves as a cleaning gas for solar panel production and liquid crystal displays (LCDs). Emissions can be reduced by using fully enclosed systems.
[1] https://pure.iiasa.ac.at/id/eprint/18412/1/THG-Budget_Hintergrundpapier_CCCA.pdf [2] https://fis.uni-bamberg.de/bitstream/uniba/53740/3/fisba53740_A3a.pdf [3] https://essd.copernicus.org/articles/14/4811/2022/ Kluthe Magazine
Kluthe Magazine

