« Separating Solvents and Other Applications of Fractionation Methods »
In chemistry, fractionation refers to the process of separating mixtures into their individual components. This is achieved using a variety of specialized chemical separation techniques. The term “fractionation” is typically used when mixtures consist of more than two substances that need to be isolated individually or in groups. Here you will find an overview of the most important methods and their areas of application.
Natural Phenomena Commonly Used in Fractionation
Chemistry often utilizes physical methods to separate mixtures. These methods harness natural phenomena such as:
- Changing states of matter at different temperatures (e.g., distillation)
- Differing solubilities of substances (e.g., extraction, precipitation, absorption)
- The natural tendency toward concentration equilibrium (e.g., diffusion, osmosis)
- Selective accumulation of substances on the surface of solids (adsorption)
- Varied permeability of particles or molecules through porous solids (e.g., filtration, ultrafiltration)

Typically, these phenomena lead to equilibrium states where differences are balanced. In chemical fractionation, the intentional adjustment of process parameters such as concentration, temperature, pressure, shifts the equilibrium toward the desired outcome. Mixtures then form distinct phases with substances accumulating differently between each phase or concentrating at the interfaces. Phases may differ by state of matter (solid, liquid, gas) or by immiscibility (such as oil and water). They often separate spontaneously: gases escape from liquids and can be collected, solids settle as sediment, and immiscible liquids form distinct layers. If needed, fractionation is completed using filters, separators, or centrifuges.

Conducting Fractionation
Fractionation in chemistry typically involves sequentially applying different separation methods. For example, solids may first be filtered from a liquid mixture, followed by precipitating dissolved salts, filtering them out, and finally separating the remaining liquid mixture into its components through distillation processes. Each step produces two new fractions, either final products or further processed materials. Examples include:
- Solvent extraction of plant oils from biomass
- Fractional distillation of crude oil or solvent mixtures
- Adsorption of organic pollutants onto activated carbon for wastewater treatment
- Adsorption of water vapor onto silica gel for air drying
- Reverse osmosis for seawater desalination

How Solvent Extraction Recovers Plant Oils from Biomass?
When extracting plant oils from biomass, oil-rich plant parts are mixed with petroleum ether or alcohol. The oils dissolve in the solvent. After the solids are filtered out, the solvent-oil mixture is distilled, resulting in pure oil. Small amounts of oil evaporate with the solvent; this vapor mixture condenses in a cooler and can be reused. Extraction, which involves selectively dissolving components, is frequently used in chemical fractionation. Liquid mixtures are combined with solvents that dissolve the target substances but remain immiscible with other components. This process creates two separate liquid phases, allowing the separation of the solvent and the extracted material.

Fractional Distillation of Crude Oil or Solvent Mixtures
In chemistry, solvent mixtures containing more than two substances are often produced. Such mixtures also occur in surface technology, for example in industrial parts cleaning — more specifically, in solvent-based parts cleaning. Fractional distillation commonly separates these solvents, exploiting differences in vapor pressures. Lower-boiling substances concentrate more in vapor than in liquid phases. As a liquid mixture evaporates, its boiling temperature gradually rises, introducing heavier-boiling substances into the vapor. By periodically collecting and condensing the vapors separately, different fractions containing fewer components from the original mixture are obtained.
Technical Implementation of Fractional Distillation
Fractional distillation is typically performed in distillation columns—tall, vertical steel towers with evaporators at the bottom and condensers at the top. Columns usually contain internal packing materials. As vapor rises, some condensate trickles downward. Constant mass transfer between vapor and liquid phases occurs. Vapor accumulates lighter-boiling components, while heavier substances concentrate in the liquid. Individual fractions are collected at various heights along the column from the liquid phase.

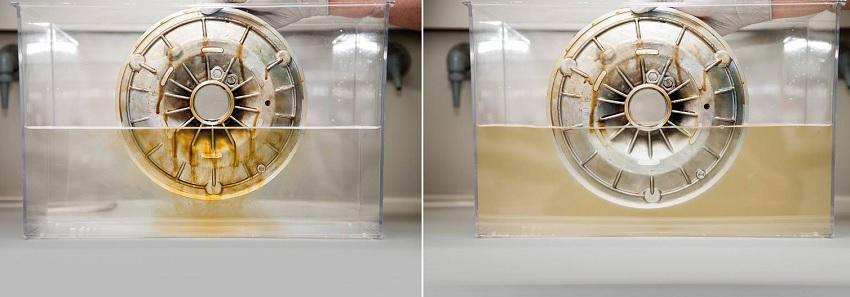
Adsorption of Organic Pollutants onto Activated Carbon for Wastewater Treatment
Activated carbon has the property of retaining organic substances on its surface. This is utilized, for example, in wastewater treatment in chemistry. Activated carbon typically exists as granules, maximizing surface area and adsorption capacity. Water percolates through or is mixed with activated carbon before filtering. Once carbon capacity is exhausted, it can be regenerated with steam, allowing the newly separated solvents to be recovered.

Adsorption of Water Vapor onto Silica Gel for Air Drying
Fractionation also includes air drying by adsorption of moisture onto silica gel or other drying agents. These materials have a high capacity for absorbing water vapor, preventing condensation and moisture-related damage during temperature fluctuations. Small packets of drying agents, such as silica gel, are commonly included with leather goods and metal products during transport to prevent corrosion.
Reverse Osmosis for Seawater Desalination
Membrane-based methods are also frequently used in fractionation. Reverse osmosis for seawater desalination is a prominent example. Osmosis naturally balances concentration differences, causing ripe cherries to burst after rain, for instance. When a concentrated solution (e.g., juice) and water are separated by a semi-permeable membrane allowing only water molecules to pass through, the water moves into the solution, diluting it and increasing internal pressure. When equilibrium is reached, the process stops. In reverse osmosis, applying pressure greater than the equilibrium pressure to the concentrated side forces water through the membrane, enabling the collection of purified water.

 Kluthe Magazine
Kluthe Magazine
