Since the mid-19th century, carbon dioxide levels in the atmosphere have steadily increased. With industrialization and rising carbon dioxide emissions, concentrations have surged from an average of 280 ppm (parts per million, a unit measuring the concentration of gases in the atmosphere) to over 400 ppm compounding increasingly evident climate changes. Of particular concern is the accelerated accumulation of CO2, suggesting that climate change is becoming self-perpetuating. To counteract this, industrial measures must be implemented to reduce greenhouse gases, making sustainability in the chemical industry increasingly important. However, reducing CO2 emissions alone is not enough; removing carbon dioxide from the atmosphere is also essential. But what options are available for CO2 binding? Read on to learn more.
How Can CO2 Be Captured?
Carbon dioxide binding can be achieved through both biological and technical methods.
Biological Methods
Biological carbon binding relies on photosynthesis, the basis of plant growth. However, CO2 captured by plants is often re-released into the atmosphere through natural decomposition. Thus, plants can only serve as long-term carbon storage if additional steps are taken to prevent biomass breakdown.
Technical Methods
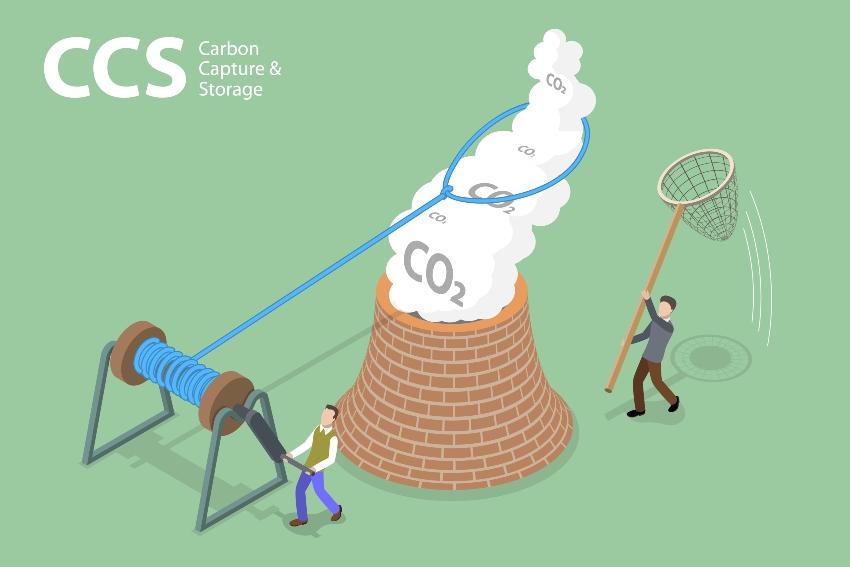
Technical methods for carbon dioxide binding are closely linked to the capture of CO2 from the atmosphere or industrial emissions. Permanent carbon dioxide binding is then achieved through utilization or storage. The following terms and abbreviations have become standard for these processes:
- Direct air capture of CO2 (DACC)
- Carbon capture and utilization (CCU)
- Carbon capture and storage (CCS)
Plant-Based CO2 Binding Methods
How Much CO2 Does a Tree Capture?
Trees primarily bind CO2 in the wood of their trunks, branches, and roots. The amount stored depends on factors like tree species, growth conditions, and development stage, which influence the amount of wood produced. About half of the dry wood’s mass is carbon; by multiplying this value by 3.67, we can estimate the mass of carbon dioxide the tree has absorbed from the air.
For those who want to calculate: 12 g carbon + 2*16 g oxygen = 44 g CO2; 44 : 12 = 3.67.
You can find the atomic masses in the periodic table of elements.
A more informative metric is the amount of greenhouse gas that can be captured through reforestation. A general rule of thumb is that forests can sequester about six metric tons (or approximately 13,200 pounds) per 2.5 acres each year. However, this applies only to forests with healthy trees of all developmental stages growing under favorable climatic conditions. Water is also required for wood formation, so in the absence of rain, growth is delayed. Sustainable forest management and reforestation is necessary to replace what has been used in commercial industries such as; construction, furniture production and in other consumer goods, for long-term CO2 binding in forests.

CO2 Binding in Biomass
There are several effective ways for biomass—such as crops, plant residues, or algae—to bind CO2:
- Peatland restoration: where peat forms in oxygen-free conditions from biomass and remains in the soil as a carbon dioxide sink.
- Biochar production: by heating under oxygen-free conditions (similar to charcoal production), which can be used as a raw material in the chemical industry or for soil improvement in agriculture.
- Humus formation as a soil carbon sink
- Production of chemicals from biomass
- Combustion of dried biomass for energy generation, with CO2 capture and storage or utilization from flue gases
- Production of biofuels to reduce emissions from fossil fuel sources

Technical Methods for CO2 Binding
Direct Air Capture
Direct capture is very costly due to the relatively low concentration of CO2 in the air. Large fans with high air throughput are required to achieve significant yields. Several methods are available for capture:
- Gas scrubbing with liquid organic amines or caustic soda
- CO2 binding through adsorption onto a solid (sorption)
- Binding by anion exchangers with polymer resin
- Membrane technology to separate carbon dioxide from air.
The economic feasibility of these methods depends on the extent to which captured CO2 can be used. Factoring in the damage caused by global warming could shift the economic calculation significantly in favor of carbon dioxide capture.
Capture from Combustion and Industrial Emissions
With a higher concentration of CO2, capturing emissions from combustion gases is more cost-effective than air capture. Gas scrubbing with liquid organic amines is the preferred method here. Additionally, significant amounts of CO2 are produced in industries like cement production, metallurgy, and other chemical processes, which can be captured, separated, and utilized for long-term binding, much like CO2 captured from the air.

Sustainable Binding: CO2 Utilization Options
Production of Chemicals and Fuels
CO2 can be catalytically converted into basic chemicals and further processed. Examples of raw materials include urea and methanol. End products might include plastics or synthetic lubricants that serve as storage. Additionally, it is possible to produce fuels and energy sources. Although this does not reduce CO2 levels in the air—since burning the materials releases CO2 again—it reduces emissions through a circular economy approach.
Enhanced Weathering
CO2 can be catalytically converted into basic chemicals and further processed. Examples of raw materials include urea and methanol. End products might include plastics or synthetic lubricants that serve as CO2 storage. Additionally, it is possible to produce fuels and energy sources. Although this does not reduce CO2 levels in the air—since burning the materials releases CO2 again—it reduces emissions through a circular economy approach.
CO2 Binding in Concrete Building Materials
Concrete materials contain calcium hydroxide in their pores, which reacts with atmospheric CO2 to form calcium carbonate, strengthening the concrete. This natural aging process enables concrete to function as a carbon dioxide sink. During concrete recycling from demolished buildings, this process can be exploited by exposing crushed concrete to CO2 compacts the structure and reduces pore volume.
 Kluthe Magazine
Kluthe Magazine

